Atomic Structure & The Periodic Table
Chemistry delves into the world of matter, its composition, and its transformations. At the heart of this exploration lies the atom, the fundamental building block of all matter. This chapter embarks on a journey into the fascinating realm of atomic structure, exploring the subatomic particles that constitute atoms and their organization within the periodic table. We will delve into the concepts of atomic number, mass number, isotopes, and periodic trends, unveiling the underlying principles that govern the organization and behavior of elements.
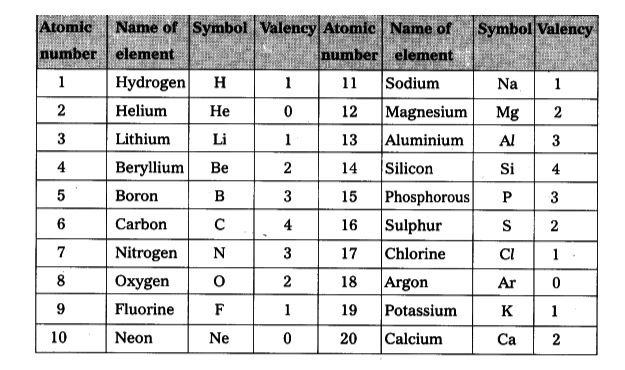
First 20 Elements Chart
Atom:
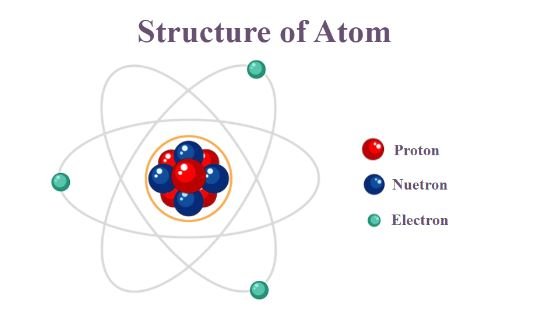
Atoms are incredibly small particles, existing in vast numbers yet invisible to the naked eye. Despite their minute size, they possess a complex structure. Here are the key subatomic particles that make up an atom:
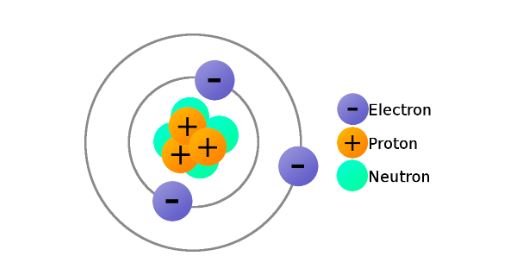
- Protons: Protons are positively charged particles located within the nucleus, the central core of the atom. The number of protons in an atom’s nucleus defines its atomic number (Z), a unique identifier for each element.
- Neutrons: Neutrons are electrically neutral particles also found within the nucleus. They contribute to the overall mass of the atom but do not affect its atomic number.
- Electrons: Electrons are negatively charged particles that reside in orbitals around the nucleus. Electrons exist in specific energy levels, and their arrangement dictates the chemical properties of the element. The total number of electrons in an atom typically equals the number of protons (except in ions, which have gained or lost electrons).
The number of protons and neutrons within an atom determines its mass.
Atomic Number, Mass Number, and Isotopes:
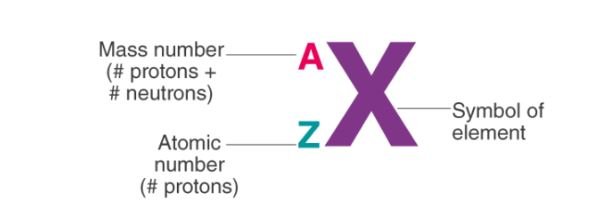
- Atomic Number (Z): As mentioned earlier, the atomic number (Z) is the number of protons in an atom’s nucleus. It is a unique identifier for each element and determines the element’s chemical identity. No two elements can have the same atomic number.
- Mass Number (A): The mass number (A) represents the total number of protons and neutrons in an atom’s nucleus. It is calculated by adding the number of protons (Z) and the number of neutrons (N). (A = Z + N)
- Isotopes:
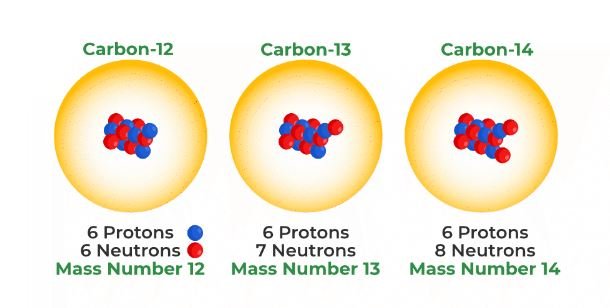
Atoms of the same element can have varying numbers of neutrons, resulting in isotopes. Isotopes have the same atomic number (Z) but different mass numbers (A) due to the difference in neutrons. For example, carbon-12 (¹²C) has 6 protons and 6 neutrons, while carbon-14 (¹⁴C) has 6 protons and 8 neutrons. Some isotopes are radioactive, meaning their nuclei decay over time, releasing energy and particles.
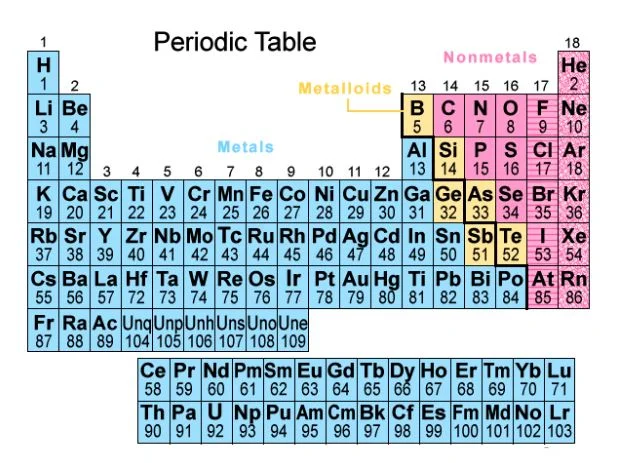
The Periodic Table
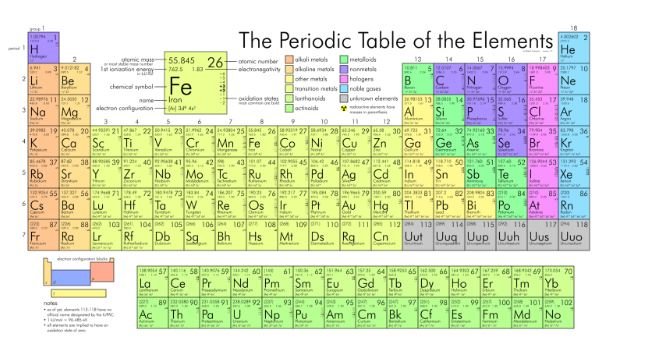
The periodic table is a powerful tool that organizes all known elements based on their atomic number, electron configuration, and recurring chemical properties. Elements are arranged in rows (periods) and columns (groups) with elements sharing similar chemical behaviors grouped together.
Here’s a breakdown of the key features of the periodic table:
- Periods (Rows): Elements in the same period (horizontal row) have the same number of electron shells (energy levels) around the nucleus. As you move from left to right across a period, the atomic number and the number of electrons increase.
- Groups (Columns): Elements in the same group (vertical column) share similar outermost electron configurations (valence electrons) and exhibit similar chemical properties. For example, Group 1 (alkali metals) elements readily lose one electron to form positively charged ions (cations), while Group 17 (halogens) elements tend to gain one electron to form negatively charged ions (anions).
Metals, Nonmetals, and Metalloids: The periodic table can be broadly divided into metals, nonmetals, and metalloids.
- Metals: Generally located on the left side of the table, metals are good conductors of heat and electricity, malleable (can be hammered into thin sheets), and ductile (can be drawn into wires).
- Nonmetals: Found on the right side of the table, nonmetals are typically poor conductors of heat and electricity. They exist in various states (solid, liquid, or gas) at room temperature and exhibit diverse properties.
- Metalloids: These elements lie along a diagonal line separating metals and nonmetals. They exhibit properties of both metals and nonmetals.
Periodic Trends
As you move across the periodic table, certain properties of elements exhibit predictable trends. Here are two key periodic trends:
-
Atomic Radius:
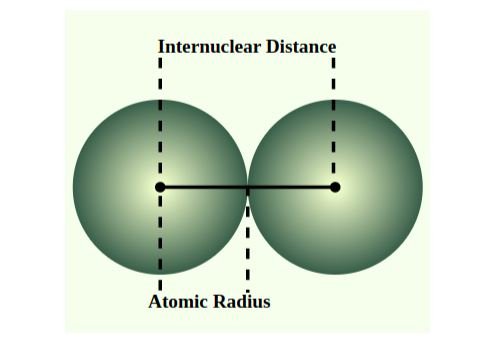
Atomic radius refers to the half-distance between the nuclei of two bonded atoms. Generally, atomic radius decreases as you move from left to right across a period. This is because the effective nuclear charge increases as you move from left to right across a period due to the increasing number of protons attracting the electrons. The increased attraction pulls the electrons closer to the nucleus, resulting in a decrease in atomic radius. Conversely, atomic radius increases as you move down a group (column). This is because additional electron shells are added as you move down a group, increasing the distance between the outermost electrons and the nucleus.
-
Electronegativity:
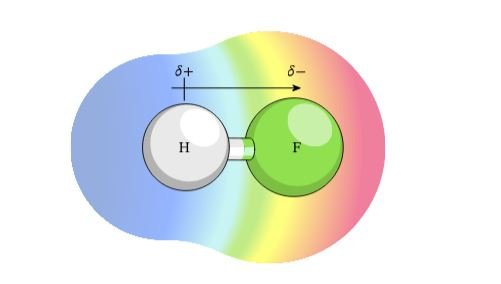
Electronegativity refers to the relative ability of an atom in a molecule to attract electrons towards itself. It is a measure of an atom’s electron-withdrawing power. Electronegativity generally increases as you move from left to right across a period. This trend follows the same logic as atomic radius – the increasing effective nuclear charge draws electrons closer, making them harder for other atoms to attract. Electronegativity tends to decrease as you move down a group. The additional electron shells in lower groups shield the nucleus from the outermost electrons, making them slightly less attracted to the nucleus and easier for other atoms to pull in.
Understanding these periodic trends allows you to predict and explain the chemical behavior of elements based on their position in the periodic table. For example, knowing that fluorine (F) has the highest electronegativity on the periodic table helps us understand why it readily gains an electron to form a fluoride anion (F⁻) and is highly reactive with other elements.
Additional Points to Consider
-
Electron Configuration:
Electrons occupy specific energy levels or orbitals around the nucleus. Understanding electron configuration, how electrons are distributed among these orbitals, is crucial for predicting chemical behavior and bonding.
-
Valence Electrons:
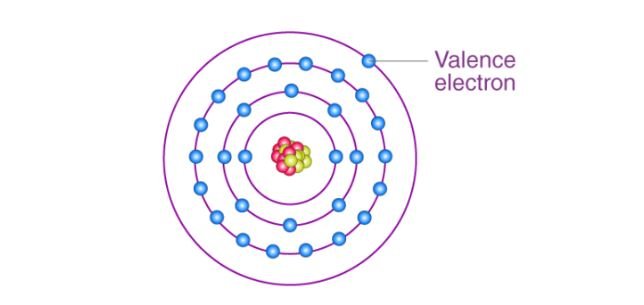
The outermost electrons in an atom’s orbitals are called valence electrons. They are the most important electrons for determining an element’s chemical properties and its bonding behavior with other elements.
-
Ionization Energy:
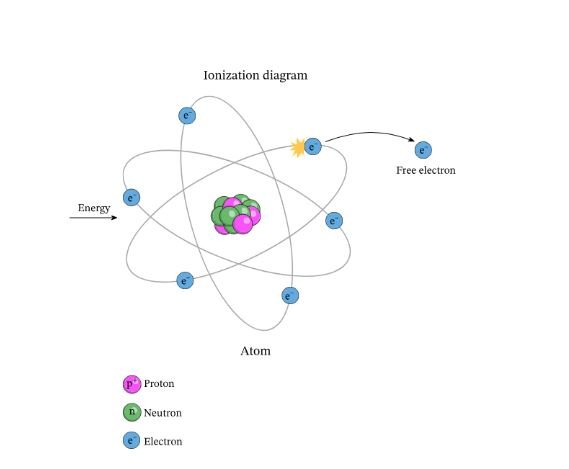
The energy required to remove an electron from an atom or ion is called ionization energy. Ionization energy generally increases as you move from left to right across a period and decreases as you move down a group.
By delving into atomic structure, atomic number, mass number, isotopes, the periodic table, and periodic trends, you gain a fundamental understanding of the building blocks of matter and the principles that govern their organization and behavior. This knowledge forms the foundation for further exploration of chemical bonding, reactions, and the fascinating world of chemistry.
