Chemical Bonding & Nomenclature
Chemistry is the story of how atoms interact and transform. This chapter delves into the fascinating world of chemical bonding, the force that holds atoms together to form molecules and compounds. We will explore the three main types of chemical bonds – ionic, covalent, and metallic – and delve into Lewis structures, which depict the arrangement of atoms and electrons in molecules. We will then explore how chemical formulas represent compounds and the systematic approaches to naming ionic and covalent compounds.
Ionic, Covalent, and Metallic Bonds
The type of chemical bond formed between atoms depends on their electronegativity, the relative ability of an atom to attract electrons towards itself.
-
Ionic Bonding:
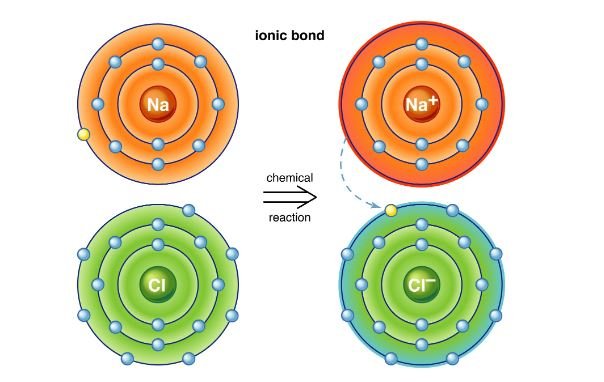
Ionic bonds are formed between a metal and a nonmetal with a significant difference in electronegativity. In ionic bonding, the metal donates one or more electrons to the nonmetal. The resulting charged particles are called ions – the metal loses electrons to become a positively charged cation, and the nonmetal gains electrons to become a negatively charged anion. The electrostatic attraction between the oppositely charged ions holds the compound together. For example, in sodium chloride (NaCl), sodium (metal) loses an electron to become Na⁺, and chlorine (nonmetal) gains an electron to become Cl⁻. The electrostatic attraction between Na⁺ and Cl⁻ forms the ionic bond in sodium chloride.
-
Covalent Bonding:
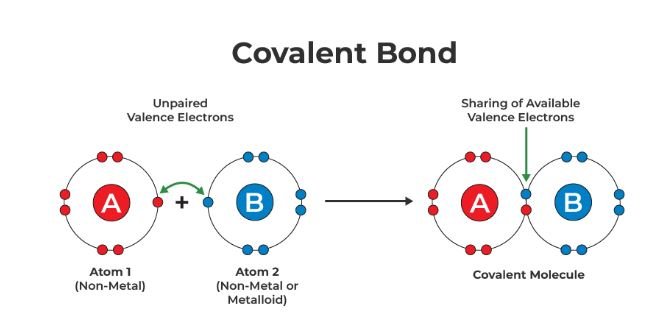
Covalent bonds are formed when two nonmetals share electrons. Atoms participating in a covalent bond achieve a stable configuration similar to a noble gas (full valence shell) by sharing electrons. Covalent bonds can be single, double, or triple, depending on the number of electron pairs shared between the atoms. For example, in methane (CH₄), each hydrogen atom shares its only valence electron with the carbon atom, and the carbon atom shares one electron with each hydrogen atom to achieve a full valence shell.
-
Metallic Bonding:
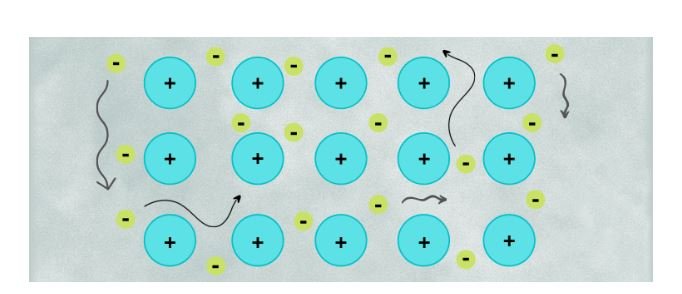
Metallic bonding is a specific type of bonding that exists in metals. In metallic bonding, metal atoms lose their valence electrons, forming a positively charged sea of nuclei and delocalized electrons. The delocalized electrons are free to move throughout the metal, creating a strong electrostatic attraction that holds the metal cations together. This “sea of electrons” model explains the characteristic properties of metals, such as good conductivity of heat and electricity.
Lewis Structures: Unveiling the Architecture of Molecules
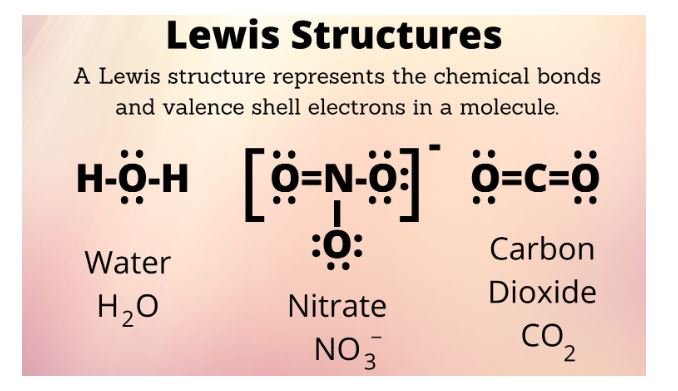
Lewis structures, also known as electron dot structures, are a visual representation of a molecule that shows the arrangement of atoms and the distribution of valence electrons. Lewis structures are helpful for understanding covalent bonding and predicting molecular geometry.
Here are the key steps for drawing Lewis structures:
- Determine the central atom: In most molecules, the central atom is the least electronegative element.
- Count the total valence electrons: Add the number of valence electrons from each atom in the molecule.
- Form single bonds: Draw single bonds (one shared electron pair) between the central atom and each bonded atom.
- Satisfy octet rule: Place electrons around each atom to achieve an octet (eight electrons in the valence shell) except for hydrogen, which needs only two electrons.
- Formal charges: If the octet rule cannot be satisfied by placing all electrons as lone pairs (unshared electron pairs) on terminal atoms, some electrons may need to be moved to form double or triple bonds with the central atom. Formal charges are assigned to represent the difference between the number of valence electrons an atom has in the free state and the number of electrons it has in the Lewis structure.
By understanding Lewis structures, you can predict the geometry of simple molecules using the VSEPR (Valence Shell Electron Pair Repulsion) theory. VSEPR theory states that electron pairs (bonding and lone pairs) around a central atom will repel each other and adopt an arrangement that minimizes repulsion, resulting in a specific molecular geometry.
Chemical Formulas and Naming Conventions: A Language for Compounds
Chemical formulas represent the composition of a compound using symbols and numbers. There are two main types of chemical formulas – empirical formulas and molecular formulas.
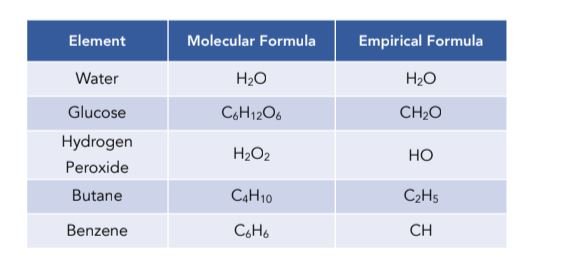
- Empirical Formula: The empirical formula shows the simplest whole-number ratio of atoms in a compound.
- Molecular Formula: The molecular formula indicates the exact number of atoms of each element present in a molecule.
Chemical nomenclature is a systematic way of naming ionic and covalent compounds. Here’s a breakdown of the naming conventions for each type:
- Ionic Compounds: Ionic compounds are named by cation first, followed by the anion name with the ending changed to -ide (except for some common anions like oxide (O²⁻), sulfide (S²⁻), and nitride (N³⁻)). Roman numerals are sometimes used to indicate the charge of the cation if the element can exhibit multiple oxidation states (number of electrons lost by an atom to form a cation). For example, FeCl₂ is named iron(II) chloride, and FeCl₃ is named iron(III) chloride.
- Covalent Compounds: Covalent compounds are typically named using prefixes to indicate the number of atoms of each element present in the molecule. The prefixes used are: mono- (1), di- (2), tri- (3), tetra- (4), penta- (5), hexa- (6), hepta- (7), octa- (8), nona- (9), and deca- (10). The first element’s name remains unchanged, while the second element’s name takes the ending -ide. For example, CO₂ is named carbon dioxide, and N₂H₄ is named dinitrogen tetrahydride (often shortened to hydrazine).
Polyatomic Ions: Some covalent groups of atoms act as a single unit with a charge and are named polyatomic ions. When naming ionic compounds containing polyatomic ions, the cation is named first, followed by the polyatomic ion name. For example, CaCO₃ is named calcium carbonate, and NaNO₃ is named sodium nitrate.
Exceptions: There are some exceptions to these naming conventions for common covalent compounds like water (H₂O) and ammonia (NH₃).
By understanding chemical formulas and naming conventions, you can decipher the composition of a compound from its formula and name a compound based on its chemical structure.
Additional Points to Consider
- Octet Rule Exceptions: While the octet rule is a helpful guideline for Lewis structures, there are some exceptions. For example, some elements like boron (B) and beryllium (Be) can only accommodate six electrons around their central atom in stable Lewis structures.
- Resonance Structures: In some molecules, where multiple valid Lewis structures can be drawn that differ in the placement of electrons but not the atomic arrangement, resonance structures are used to represent the delocalization of electrons throughout the molecule.
- Isomers: Isomers are compounds that have the same chemical formula but different arrangements of atoms. There are two main types of isomers – structural isomers (different bonding arrangements) and stereoisomers (same bonding arrangements but different spatial arrangements of atoms).
Understanding these additional concepts will further enhance your grasp of chemical bonding and nomenclature, allowing you to tackle more complex molecules and chemical phenomena.
